Supporting Documents:
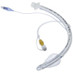
The need to reduce ventilator associated events is the primary reason the Shiley Evac Oral Endotracheal Tube with TaperGuard Cuff is critical for all patients on a ventilator in the ICU. The use of the Shiley reduces Ventilator-Associated Pneumonia (VAP) by an Average of 50%.
Subglottic secretion drainage (SSD) helps remove oral and/or gastric secretions from above the endotracheal tube cuff before they can be aspirated. Such aspirations may lead to a very serious complication known as VAP or Ventilator Associated Pneumonia.
SSD must be done with a specialized endotracheal tube with a separate dorsal suction lumen located just above the cuff. Based upon clinical evidence, the following organizations recommend the use of SSD to reduce the incidence of VAP:
Shiley Evac Oral Endotracheal Tube with TaperGuard Cuff Features:
The use of subglottic suctioning combined with the hospital-designed VAP or VAC bundle can:
There are currently no reviews for this product. Pease write a review by clicking the button below.