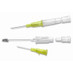
Supporting Documents:
The B | Braun Introcan Safety IV Catheter is a passive safety device that is activated automatically and cannot be bypassed. This easy-to-use safety IV catheter incorporates separate needle and catheter flashback with Double Flashback technology that offers separate confirmation of both needle and cather placement, which provides the added confidence of successful placement every time. The sharp needle features Universal Bevel geometry that allows a wider choice of insertion angles, offering greater adaptability. As an added benefit, these catheters also have less plastic content than other safety IV catheters, which can potentially reduce storage space and medical waste.
B | Braun Introcan Safety IV Catheters Feature:
- Reduces needlestick injuries with a safety mechanism is always activated and prevents re-insertion of the stylet
- Promotes first-stick success with the inclusion of Double Flashback technology for confirmation of both needle and catheter placement individually
- New Thinwall Technology is included 24G size catherters, which speeds the flashback of blood to assist in the confirmation of correct placement
- Universal Bevel geometer offers a wider choice of insertion andles for greater patient comfort and reduced risk of tearing
- Lighter, more compact design can potentially reduce storage and waste disposal costs
- Latex Free
There are currently no reviews for this product. Pease write a review by clicking the button below.